重金属:修订间差异
imported>Manchiu Cat-a-lot:從分類移除:Category:重金属 |
(没有差异)
|
2024年8月10日 (六) 01:09的最新版本
Template:NoteTATemplate:CJK-New-Char Template:About Template:Multiple image 重金属是一类金属元素的统称,通常代表密度大于5 g/cm3的金属,但在不同情况下有許多種不同的定義[1]。重金属概念的出现,是由于一系列密度较小的金属的发现,进而金属被逐渐分为轻重两类。而根据领域不同,除密度之外还存在基于原子序数和化学性质等方面的定义。重金属元素在宇宙中经核聚变和中子俘获产生,不同的重金属元素在地壳中的丰度和富集程度不同,因此需要使用不同的开采和提纯方式来生产。
许多重金属元素因其密度、强度、电磁和化学特性在众多领域都有所应用,例如工程、医疗、军事等。而对于生物,一些重金属元素对于生命活动必不可少,例如氧气运输、辅因子、葡萄糖利用等方面均需要一定量的重金属元素参与。而有些元素则对生物有不同程度的毒性。工业活动所导致的重金属元素泄漏造成了包括水俣病事件在内的多次重金属生态灾难事件。
定义
| 重金属在元素周期表上的位置 | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | |||||||||||
| 1 | H | He | ||||||||||||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | ||||||||||||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | ||||||||||||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||||||||
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||||||||||
| 6 | Cs | Ba | width="5%" Template:Element cell-asterisk | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||||||||
| 7 | Fr | Ra | Template:Element cell-asterisk | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||||||||
| Template:Element cell-asterisk | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | ||||||||||||||
| Template:Element cell-asterisk | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | ||||||||||||||
| ||||||||||||||||||||||||||||
| 各个金属元素所满足的不同“重金属判定标准”数量。本节内罗列的10个标准中,有2个基于密度,3个基于原子质量,2个基于原子序数,3个基于化学性质[註 1]这说明除了汞、铅和铋外,其他金属是否属于“重金属”还存在争议。 锗、砷、硒、锑、碲和砹一般而言被视为非金属,而在此处也计入金属[15][註 2]。Og在此处属于非金属。虚线内的元素单质密度(对于从砹至Ts的元素为预测值)大于5 g/cm3。 | ||||||||||||||||||||||||||||
“重金属”一词所包含的元素种类并无定论,其在不同语境下可能存在不同的定义。尽管如此,这个术语仍旧经常出现在科学文献中。2010年,有研究显示这个术语的使用量日益增加,似乎已经成为科学语言的一部分[16]。由于“重金属”一词既方便又为人熟悉,所以只要在使用时附上定义,这个术语也是可以接受的[17]。
通常而言,对重金属的范围进行界定时,会使用特定的理化性质进行定义。例如在冶金行业中一般依据元素单质的密度进行判定[18];在物理学中,探讨核物理时会使用元素的原子序数作为依据[19];在化学中,元素的化学性质则会成为是否将其归入重金属的考量[9]。即便是同一种理化性质,也存在不同的重金属定义。例如以密度来区分轻重金属时,存在3.5 g/cm3至7 g/cm3的多种分界线[2];以原子质量为参考时则有22.98au(钠)、40(钪)[3][註 3]、200(汞)等;以原子序数为标准时有20(钙)[2]等。
以密度判定
密度是一种常见的用来区分重金属和其他金属的性质。密度是否大于5 g/cm3是界定一个金属元素是否属于重金属的通用指标[20]。符合这个标准的类金属(例如砷和锑)有时候也会视作重金属(尤其是在环境化学领域)[21],本条目亦然。硒的密度为4.8 g/cm3[22],稍微低于上述标准,虽然较少被视为类金属[15],但其部分化學性质与砷和锑相近[23]。有些密度低于上述标准的金属在一些情况下也会当成“重金属”看待,例如铍[24](密度为1.8 g/cm3)[25]、铝[24](2.7 g/cm3)[26]、钙[27](1.55 g/cm3)[28]、钡[27](3.6 g/cm3)[29]。[註 4]
与重金属相对应的术语是“轻金属”,Template:Link-en建议用“轻金属”指称铝、镁、铍、钛、锂以及其他高活性金属[30],它们的密度介乎0.534 g/cm3至4.54 g/cm3。
以原子序数判定
原子序数亦可用于判定重金属元素,例如定义原子序数为21(钪)至92(鈾)范围内的金属为重金属。[5]使用原子序数作为标准时一些密度较低的金属可能也会被认定为重金属。例如属于碱金属的铷,其原子序数为37,而密度仅为1.532 g/cm3,低于绝大多数基于密度的标准[31]。基于原子质量的标准也有类似的情况[32]。
以化学性质判定
《美国药典》曾经提出一个辨别重金属元素的方法,原理是利用部分金屬的硫化物為有色沈澱物這一性質[6]。化学家斯蒂芬·霍克斯(Template:Lang)于1997年提出,“重金属”一词应适用于“硫化物不溶于水、氢氧化物不溶于水、盐溶于水并生成有色溶液、配合物通常有色的金属”。在这个基础上,霍克斯提出把“重金属”定义为“所有在元素周期表里位于3族至16族、同时位于第4周期或以下的金属”,即是过渡金属或貧金屬。[9][註 5]镧系元素都满足霍克斯的表述,而锕系元素则尚未确定。
在生物化学领域,重金属的定义有时建基于酸碱电子理论,以某一金属元素的离子在水溶液的路易斯酸行为(接收电子对)来区分。这个准则把较倾向接收氧原子电子对的金属离子称为“A类”,较倾向接收氮原子或硫原子电子对的金属离子称为“B类”;有些金属离子在不同情况下分别展示出A类和B类的特性,模棱两可,处于临界位置。由此,这个准则把“重金属”一词定义为上述的B类金属或临界金属。[17][註 6]A类金属包括碱金属、碱土金属、铝、3族元素、镧系元素和锕系元素,它们倾向有较低的电负性,并倾向形成离子键成分较大的化学键。[註 7]B类金属通常具有较高的电负性,并倾向于形成共价键成分较大化学键。B类金属主要是较重的过渡金属和贫金属。临界金属主要由较轻的过渡金属和贫金属组成(以及砷、锑)。三类金属中A类金属和另外两类金属的差异较大[40]。1980年,Nieboer和Richardson两人提出使用上述分类方法,以替代轻重金属分类[41]。而该提议尚未被广泛接受[42]。
词语由来
史前時代的人就已经注意到铁、金、银等Template:Link-en的密度很大。它们因延展性优良而常被制成饰物、工具或武器。[43]因为1809年之前发现的金属,密度都相对较高,所以密度曾被视为判断某材料是否为金属的唯一标准[44]。
1809年起,科学家成功提取诸如钠、钾、锶之类,密度相较以往发现金属更低的金属。这冲击了当时“金属密度都很高”的传统观点,因此有科学家提议把这些金属称作“类金属”,意指它们的形态或外观近似于金属[45][46]。但科学界最终并未采用此说法,而是承认这些元素为金属元素,而以“类金属”指称非金属元素(后又改为指代同时具有金属和非金属性质的元素)[47]。
“重金属”一词最早可追溯到1817年,德国化学家利奧波德·格梅林将化学元素分类为非金属、轻金属、重金属三种[48],其中轻金属界定为密度介于0.860 g/cm3至5.0 g/cm3的金属元素,重金属则界定为密度介于5.308 g/cm3至22.000 g/cm3的金属元素[49][註 8]。此后多年间,不同的科学家使用的重金属定义各有不同,而在一些文献中“重金属”一词也被用来称呼具有高原子量或高原子序数的元素[31]。
苏格兰毒理学家约翰·达弗斯(John Duffus)在2002年审视了过往60年“重金属”一词在学术界的定义,发现各个定义迥然不同,因而批评这个术语无意义可言[50]。学术界也经常质疑一些金属是否属于重金属,理由包括其密度过小、参与生物过程及甚少构成Template:Link-en等,例如:钪(密度过小)[31][51];钒、锌(参与生物过程)[52];铑、铟、锇(太稀有)[53]。
生物角色
| 元素 | 毫克[54] | |
|---|---|---|
| 铁 | Template:Bartable | |
| 锌 | Template:Bartable | |
| 铅[註 9] | Template:Bartable | |
| 铜 | Template:Bartable | |
| 锡[註 10] | Template:Bartable | |
| 钒 | Template:Bartable | |
| 镉 | Template:Bartable | |
| 镍[註 11] | Template:Bartable | |
| 硒 | Template:Bartable | |
| 锰 | Template:Bartable | |
| 其他[註 12] | Template:Bartable | |
| 总计 | 7000 | |
Template:See also 一些在生物体内发生的过程需要微量重金属(多数是第4周期元素)方能进行。这些重金属包括:铁、铜(氧气运输和電子傳遞)、钴(复合物合成与细胞新陈代谢)、锌(羥基化)、钒和锰(酶的调节和运作)、铬(葡萄糖利用)、镍(Template:Link-en)、砷(一些动物的代谢生长,可能包括人类)和硒(抗氧化功能和激素合成)等。[59][60]第5和第6周期中属于必要元素的重金属元素较少,符合“元素越重就越稀有,元素越稀有就越不常是必要营养”的一般规律[61]。生物需要钼(第5周期元素)或镉(某些海洋矽藻)来催化体内的氧化还原反应。有些矽藻物种需要同属第5周期的锡来生长。[62]一些古菌和细菌需要钨(第6周期元素)来进行新陈代谢[63]。如果生物缺乏上述任何一种属于必要的重金属元素,就可能会更容易出现重金属中毒[64](反之,如果体内有过剩的重金属,同样可能造成负面生物影响)。
一个体重為70公斤的人Template:Link-en通常有约0.01%的重金属(约为7克,其中最主要的三种元素是铁、锌和铅)、2%的轻金属(约为1.4公斤)、约98%的非金属(其中大部分是水)[65][註 13]。
有若干不属于必要元素的重金属也具有生物影响。例如锗(类金属)、镓、铟以及大多数镧系元素可以刺激代谢,而钛(有时不被视为重金属)能够促进植物生长。[66]
毒性
Template:See also 人们通常认为重金属有Template:Link-en(引发癌症或导致腦損傷、死亡等)[67]。某些重金属的确如此,不过部分重金属只是在摄取过量时才有毒,或者只在特定化学形式下才有毒性。
环境污染
铬、砷、镉、汞、铅是最具潜在危害的重金属,这是尽管它们的单质或化合物有剧毒,却被人类广泛使用,致使在自然环境中广泛分布[68]。例如六价铬(铬的一种形式)具有剧毒,毒性与气态汞相若、与多种汞化合物相若[69]。这五个重金属元素对硫有强大的亲和力,因此它们在人体里经常与巰基官能團(-SH)上的硫原子键合,从而结合到控制代谢反应速度的酶之上。硫原子与重金属原子之间的化学键抑制了酶的正常运作,令人体健康受损害,有时更可能死亡。[70]铬(六价铬)和砷是致癌物,镉会导致痛痛病,汞和铅会损害中樞神經系統。
-
铬金属晶体、
一立方厘米大的正方体铬块 -
密封在容器里的砷
-
条状的镉、
一立方厘米大的正方体镉块 -
正在倒进培养皿的汞
-
氧化了的西兰花状铅块、
一立方厘米大的正方体铅块
铅是最常见的重金属污染物[71]。在工业化地区,水生环境里的铅浓度估计为非工业化地区的两至三倍[72]。在1930至1970年代,四乙基铅(Template:Chem)被广泛用作汽油添加剂[73]。虽然北美洲国家在1996年前大规模淘汰了含铅汽油,但此前兴建的行车道附近的土壤已经留下了高浓度铅[74]。后来的研究发现,美国的含铅汽油使用率和暴力罪案率之间存在显著的统计学关联。若将考虑两者之间22年(暴力罪犯平均作案年龄)的时间差纳入考量,则暴力罪案率与铅暴露的统计曲线几乎完全相合[75]。
其他具有潜在危害的重金属环境污染物包括:锰(损害中央神经系统)[76];钴和镍(致癌)[77];铜[78]、锌[79]、硒[80]、银[81](对鱼类、植物、鸟类和其他水生动物造成內分泌扰乱、先天性障碍或一般毒害);锡(有機錫化合物损害中央神经系统)[82];锑(怀疑致癌)[83];铊(损害中央神经系统)[78][註 14][註 15]。
属于必要营养素的重金属
如果摄取过量,属于必要营养素的重金属也可能会变得有毒,一些元素已知存在一些有毒的形态。五氧化二钒(V2O5)对动物是致癌物,吸入可以引致DNA损伤[78]。高锰酸根离子(MnOTemplate:Su)对肝脏和肾脏有毒[87]。摄入0.5克以上的铁能够导致心脏衰竭,这种过量摄取通常发生在儿童身上,可能在24小时内引致死亡[78]。30百万分率浓度的四羰基镍()可以引致呼吸衰竭、脑损害、以至死亡[78]。吸入一克或以上的硫酸铜(CuSO4)可以致命,即使存活下来也可能使主要器官受到损伤[88]。硒的每日建议摄取量上限是0.45毫克,而若摄取超过建议量的十倍,达到5毫克,硒就具有剧毒[89];长期硒中毒还能够致瘫[78][註 16]。
其他
一些非必需的重金属元素具有一种或多种有毒化合物形态。曾出现过由于过量摄入锗导致肾衰竭甚至致命的记录[78]。暴露于四氧化锇则可能导致永久性眼损伤、呼吸衰竭[91]及死亡[92]。对于铟盐,如果摄入超过几毫克,会使肾脏、肝脏和心脏受到影响[93]。重要的化疗药物顺铂对肾脏及神经具有毒性[78]。过量摄入铋化合物可导致肝损伤。不可溶的铀化合物以及它们产生的电离辐射则可导致永久性肾损伤[94]。
暴露源
工业活动带来的重金属富集能降低空气、水及土壤的品质,并因此导致动植物健康状况下降[95]。可能导致重金属富集的来源包括工业垃圾、汽车尾气、铅酸蓄电池、肥料、染料、经过处理的木材[96]、老化的管道[97]以及海中漂浮的塑胶颗粒等[98]。1950年代发生于日本的水俣病事件,正是由于含汞废水排放入海,被水中生物摄入后转化为有机汞化合物,人食用这些海产品后有机汞进入人体所致[99]。巴西的班托羅德里格斯水壩災難[100]、美国的Template:Tsl[101]等,都是由于不当工业活动致使普通民众暴露于过量重金属的例子。[101]
形成、丰度、分布及提取
| 地壳中的重金属丰度及 | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 主要来源和分布情况[註 17]。 | |||||||||||||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | ||
| 1 | H | He | |||||||||||||||||
| 2 | Li | Be | B | C | N | O | F | Ne | |||||||||||
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar | |||||||||||
| 4 | K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge | As | Se | Br | Kr | |
| 5 | Rb | Sr | Y | Zr | Nb | Mo | Ru | Rh | Pd | Ag | Cd | In | Sn | Sb | Te | I | Xe | ||
| 6 | Cs | Ba | width="5%" Template:Element cell-asterisk | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | |||
| 7 | Template:Element cell-asterisk | ||||||||||||||||||
| Template:Element cell-asterisk | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | ||||||
| Template:Element cell-asterisk | Th | U | |||||||||||||||||
| Template:Legend | Template:Legend | ||||||||||||||||||
| Template:Legend | Template:Legend | ||||||||||||||||||
| Template:Legend | |||||||||||||||||||
| 分割线左边的重金属元素多为Template:Tsl;右边则多为Template:Tsl,除了金(Template:Tsl)和锡(亲石元素) | |||||||||||||||||||
重金属元素的产生与大多数元素类似,是从已存在的原子核发生核反应后形成。关于具体的反应形式,可按原子序数小于和大于铁元素进行区分。原子序数小于铁的重金属元素大部分是通过恒星核合成产生的。恒星内,从氢至硅的元素在恒星内部参与连续的核聚变反应,释放光和热,并生成较原子序数较大的元素。[105]
关于原子序数大于铁的元素,由于通过核聚变生成这些元素的过程中,吸收的能量大于放出的能量,故原子序数大于铁的重金属通常并不经由核聚变产生[106],它们主要由原子序数较小的元素发生中子俘获而来。中子俘获有两种主要的形式——R-过程与S-过程。在S-过程中,两次俘获事件之间可能间隔数年甚至数十年,这使得不稳定的原子核有充分时间发生β衰变[107]。而在R-过程中,俘获速率比衰变速率快。因此,S-过程的反应路径较为清晰,例如镉-110在恒星内被足够的中子轰击后变为镉-115,然后通过β衰变生成半衰期长达宇宙年龄30000倍的铟-115。铟-115可继续俘获中子变成铟-116,然后衰变为锡-116[105][108][註 18]。由于钋和砹的半衰期很短,他们会快速衰变为铋和铅,使得S-过程终止于铋。而在R-过程中则没有这样的反应路径。R-过程的俘获速率太快,以至于可以跳过一些不稳定的核素,从而产生更重的元素,例如钍和铀[110]。
关于这些过程所需的较轻元素及中子的来源,则与恒星的演化有关。恒星可能会在他们生命周期的末期会将一部分质量抛出,而中子星碰撞事件则抛出大量中子[111][註 19],产生了上述的中子俘获过程的发生条件,因而得以在星际空间中产生更重的元素。之后在引力的作用下,这些物质逐渐坍缩形成新的恒星和行星[113]。
按质量计算,地壳中大约含有5%的重金属,其中铁占95%。另外轻金属占约20%,非金属占约75%[102]。虽然总体含量较少,但由于造山运动、侵蚀作用及其他地质过程,重金属元素常常集中出现,从而在经济上有提取的价值[114]。
重金属元素中的一些元素属于Template:Le中的亲石元素(Lithophile elements),易与氧结合成化合物,另有一些属于亲硫元素(Chalcophile elements),易与除氧以外的氧族元素形成化合物。其中亲石元素主要是f区元素,且通常比d区元素具有更活跃的化学性质。它们与氧具有很强的化學親和性,常常出现在相对低密度的硅酸盐矿物中[115]。亲硫重金属元素一般较d区及第4至6周期的元素不活跃。在自然界中,这类元素一般出现于不可溶的硫化物矿物中。亲硫元素形成的矿物通常比亲石元素的密度大,因而在形成过程中会沉至地壳更深处,而丰度也更低[116]。
作為一种Template:Tsl,金不易与氧或硫化合成化合物[117]。在地球形成时,金与其他抗腐蚀金属倾向于熔合成高密度合金,并沉入地核,因而相对稀有[118]。一些相对而言較為不贵重的重金属(如钼、铼、锗和铂系金属等),其被认为主要是以亲铁金属的型態存在於地核、地幔和地殼中,而在地壳中则又被认为主要以亲硫金属的型態存在,其少以Template:Tsl形式存在於自然界[119]。[註 20]
地殼下的重金屬豐度通常較高,而大部分存在於鐵-硅-鎳核中。例如,鉑佔地殼的約十億分之一,而其在地核豐度則會高出近6000倍[120][121]。加州大学伯克利分校的研究认为,地核中的鈾、釷和钾元素可能通过衰變產生大量的熱量,來驅使板塊運動,並維持地球的磁場[122][註 21]。
從礦石中獲得重金屬的方式,主要是根據礦石不同種類的性質的變化而變化。提煉的方式方法會涉及到各種金屬的化學性質、方法的經濟效益、方法複雜性等等。這會導致不同的煉油廠或國家可能會使用不同的方法來提煉,所以其可能與此處所列之簡要大綱不同。
除了一些特殊例子之外,從廣義上來講,親石重金屬可以通過電解或化學處理來從其礦石中提取出來;而親硫的重金屬元素則是通過高溫煅燒原礦石中的硫化物,使其轉換成對應的氧化物,然後繼續煅燒重金屬氧化物,以獲得純金屬元素[124][註 22]。由於鐳元素於自然界存量較少,不能產生經濟效應;所以主要是從核廢料中提取出來的[127]。親硫的鉑系元素主要是與其他親硫元素熔合在一起形成的親硫原礦石。這些原礦石需要通過Template:Tsl、煅燒,再Template:Tsl於硫酸中,才能提取出不可與硫酸反應的鉑系元素。這是化學精製得到的純金屬單質[128]。與其他金屬元素相比,鉑系元素因為元素豐度[129]與生產成本[130]的原因,而導致其更為昂貴。
金元素由於屬Template:Tsl的緣故,導致在工業作業上,可以通過黃金氰化法從原礦石中提取出來[131]。於黃金氰化法的反應中,礦石中的金元素單質會形成氰化亞金離子()。
例如,將原礦石擊碎後,投入氰化鉀溶液與氧氣共同作用下,就可以得到氰化亞金鉀和氫氧化鉀的混合溶液;其化學方程式如下:
然後再在混合溶液中加入鋅,由於鋅的活躍程度遠遠高於金,可发生取代反应而将金析出。反應方程式如下:
金元素單質作為沉澱物從溶液中析出,隨後被濾出並熔化成金錠。[132]
重金属与轻金属
由于“轻金属”和“重金属”的定义目前并未达成广泛共识,因此对二者进行总体对比时应小心谨慎。而且,金属的硬度和强度随纯度、晶粒大小、预处理工序而异,差异可以很大。[133]下表笼统地总结了重金属和轻金属(及其化合物)的一些物理性质和化学性质。
| 物理性质 | 轻金属 | 重金属 |
|---|---|---|
| 密度 | 通常较低 | 通常较高 |
| 硬度[134] | 倾向柔软,容易切开或弯曲 | 多数颇硬 |
| 热膨胀程度[135] | 通常较高 | 通常较低 |
| 熔点 | 通常较低[136] | 低至极高[137] |
| 强度[138] | 通常较低 | 通常较高 |
| 化学性质 | 轻金属 | 重金属 |
| 在周期表的位置 | 常见于1族和2族[139] | 几乎全部见于3族至16族 |
| 活性[30][140] | 活性较高 | 活性较低 |
| 地球化学性质 | 轻金属 | 重金属 |
| 在地壳的丰度[102][140] | 丰度较高 | 丰度较低 |
| Template:Link-en | 亲石元素[104] | 亲石元素、亲硫元素(金是亲铁元素) |
| 生物学角色 | 轻金属 | 重金属 |
| 营养作用[141] | 部分为宏量营养素(钠、钾、镁、钙) | 部分为微量营养素(钒、铬、锰、铁、钴、镍、铜、锌、钼) |
| 物理性质 | 化合物 | 轻金属 | 重金属 |
|---|---|---|---|
| 溶解度 | 硫化物 | 可溶至不溶[註 23] | 极易溶[註 24][146] |
| 氢氧化物 | 可溶至不溶[註 25] | 通常不可溶[150] | |
| 水溶液颜色 | 盐[143] | 通常无色 | 通常有色 |
| 络合物 | 通常无色[151] | 通常有色[152] |
这些性质帮助人们较易分辨重金属(例如钨)和轻金属(例如钠),但两者之间的差异到了临界情况就变得模糊不清。钡、钪、钛等轻金属具有一些重金属的性质,例如熔点较高[註 26];锌、镉、铅等重金属(同时为贫金属)具有一些轻金属的性质,例如相对较柔软[註 27]、熔点较低[註 28] 、主要生成无色配合物。[33][35][36]
應用
重金属在生活中很常见,例如铁占所有精炼金属产量的90%[157]。一些常见的用途中,人们只是利用了重金属材料所具有的普通的金属性质,例如导电性和反光特性,或是密度、强度和耐久性度。某些重金属元素因其特性,具有一些特别的用途。例如由于部分重金属原子的 d 轨道或 f 轨道未填满,使得它们可以形成彩色的化合物[158],这包括大多数过渡金属和镧系、锕系元素。另一些重金属元素则在化合物中显示出多种氧化态,使得它们可以催化一些化学反应。具有此特性的金属包括铂[159]、铈[160]和铋[161]等。
一些元素3d或4f轨道重叠较弱,使得这些元素具有磁性,例如铁、钴、镍及铕至铥等镧系重金属[162]。而高原子序数和电子密度则使元素具有核物理应用价值[163]。重金属元素的典型用途按其所利用的性质,可以归为以下列出的六类[164]。
重量與密度

重金属材料的一个主要特点就是擁有较高的密度。在体育、机械工程、武器以及原子核物理学等领域中都有利用这个特点。潜水中,潛水者會使用铅作为配重[166];障碍赛马中,马匹需要佩戴指定重量的铅,以平衡选手的获胜概率[167],高尔夫球、球道及球杆中会使用钨、黄铜等降低重心位置,使得球手更容易將球擊打至半空[168],含钨的球可能拥有更好的飞行特性[169];飞蝇钓的钓线中使用的聚氯乙烯含有钨粉,可使钓线具擁有一定重量[170];链球和铅球則會分别使用钢和铅作为球的填充物以达到国际规则规定的重量[171]。
直至1980年,链球中都有使用钨。自1981年起,链球的最小直径变大,使得制造链球时,不再必须使用钨。当时,钨属于比较昂贵的金属,且不是在所有的国家都能买到[172]。钨制的链球密度之大,以至于常常在草皮上砸出很深的洞[173]。
在机械工程领域,重金属常用于船舶[174]、飞机[175]及机动车[176]等。轮胎平衡、曲轴[177]、陀螺仪、螺旋桨[178]、Template:Tsl[179],以及需要在极小的空间内使用大质量物体的情况(如手表Template:Tsl)[175]。
Template:Quote box 在军械方面,Template:Tsl、穿甲弹及核武器(用于提高中子反射率及延迟反应材料的膨胀)中使用了钨和铀[180][181][182]。1970年代时,研究发现钽在锥形装药及Template:Tsl中的性能优于铜,原因是它的密度更高,穿甲效果更强[183]。在一些军队及娱乐性射击活动中,会使用毒性较小的重金属(例如铜、锡、钨、铋、或许还有锰以及非金属的硼)制成的Template:Tsl代替铅制及锑制的子弹[184]。而关于钨对环境的影响则尚有争论[185]。
通常来说,材料的密度越高,越能有效吸收辐射。因此,Template:Tsl中常会用到重金属材料。而在放射線療法及直线粒子加速器中则会将重金属材料用于粒子束准直。[186]
強度與耐久度

因为一些重金属材料具有良好的强度和耐久度,所以在工具、机械[189]、家用电器[190]、铁路运输[191]、建筑[192]、桥梁[193]、汽车[190]等各个方面均有应用。重金属元素也可与其他金属制成合金,以增强其性能。[註 30]
重金属材料亦是金属货币和首饰、玩具中常用的材料。除了碳和铝之外,其余二十多种被用来铸造货币的材料都是重金属。[195][註 31]此外,首饰中会使用金、银和铂[註 32]。同样用于制作首饰的还有由金和镍、铟、钴等制成的Template:Tsl[198]。铬、镍、镉和铅则可能用于玩具和廉价首饰中。[199][200]
铜、锌、锡和铅虽然强度较弱,但具有优良的抗腐蚀特性。当它们与空气中的气体反应时,会分别生成铜绿及多种铜盐[201]、碳酸锌、二氧化锡和一氧化铅、碳酸铅、硫酸铅,发生钝化,保護其內部材料不會再受腐蝕[202]。因此,铜和铅常用来Template:Tsl[203][註 33]在熱浸鍍鋅鋼中,鋅是Template:Tsl材料[204];錫也是有抗蝕的特性,用在罐頭上[205]
鐵和鉻可以透過加入钆來降低其加工難度(workability)及提昇其抗蝕性。鎳可以加入钍來改善蠕变的問題。銅合金及鋼合金中可以加入碲改善機械加工性(machinability),也可以加入鉛使其提升其硬度和抗酸蝕能力[206]。
生物與化學

自古以来,人们就发现某些重金属的微动力学效应,可作用于杀虫剂。[208] 铂、锇、铜、钌、和其它重金属,包括砷,可以用来抗癌或有抗癌的潜能。[209] 锑(抗原生动物)、铋(抗溃疡)、金(抗关节炎)、和铁(抗疟疾)在药物化学里也是很重要的。[210] 防腐剂配方中也使用了铜,锌,银,金或汞;[211]少量的某些重金属可用于控制藻类在冷却塔中的生长。[212] 农业化学取决于其用作肥料或杀虫剂的预期用途,可能包含重金属,例如铬,钴,镍,铜,锌,砷,镉,汞或铅。[213]
一些重金属可以用来做燃料的催化剂(例如铼)、合成橡胶和制作纤维(铋)、催化轉換器(钯)及Template:Le(烤箱壁含有二氧化铈,可以氧化烤箱壁的含碳殘餘物)[214]在肥皂化工里,會用重金屬形成不溶於水的肥皂,可以用在Template:Le、油漆快乾劑和Template:Le[215]。制氯工业中使用有二氧化钌涂层的钛作为阳极[216]。 Template:Clear
著色與光學

玻璃、釉、涂料和塑料中的颜色常来自于重金属元素的离子,例如铬、锰、钴、铜、锌、锆、钼、钕、银、锡、镨、铒、钨、铱、金、铅、铀等[218]。例如刺青使用的墨水中可能含有铬、钴、镍和铜[219]。另外,在光学和天文学器件中,常在反射镜中采用某些具有高反射率的重金属材料。例如车前灯的反射面会使用铑薄膜来提高反射率[220]。
電磁與發光性質

重金属及其化合物在电子元件、电极材料、电线和光伏模组中常作为导体、半导体或绝缘体使用。印刷电路板中会使用铜粉、钼粉[221];供电系统中大量使用铜导线,是利用了铜良好的导电性[222]。电子设备中会使用银和金作为导体,尤其是接触开关处。这是由于这两种元素的导电性较好,且其表面上不易出现杂质[223]。碲化镉和砷化鎵這兩種半導體會被用於製造太陽能電池板。二氧化铪是一種絕緣體,可用作製造集成電路中的Template:Tsl。另一種絕緣體——五氧化二鉭,則是會被用於製造手機的電容器[224]。重金屬被用於製造電池已至少存在200年歷史,可以追溯到1800年亞歷山德羅·伏打使用銅和銀發明並製造的伏打电堆[225]。重金屬目前依然被用於製造各種電池,例如钷、镧镍合金[226][註 34]和汞可以分別用於製造核電池、镍氢电池和鈕扣電池。[227]
磁铁是由锰,铁,钴,镍,铌,铋,镨,钕,钆和镝等重金属制成。钕磁铁是目前已知最强的永久磁铁,在許多機械中都有應用。例如汽車中的車門鎖、Template:Le、Template:Le和Template:Le。[228]
重金属在各种照明和发光设备中多有使用,例如白炽灯、发光二极管和激光等。白炽灯中的灯丝由钨制成[229];荧光灯管中填充汞蒸汽,以利用汞光谱中的紫外光激发荧光粉发出白光[230]。氧化铟锡因其同时具有透明和导电两个特性,故可用在诸如液晶显示器、有机发光二极管和等离子体显示器之类的平板显示器中[231]。
核子與放射性
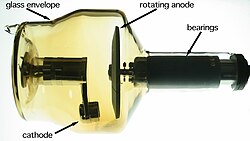
由于具有放射性,高原子序数重金属在诊断成像、电子显微镜和核能科学等领域也有特殊用途。在诊断成像中,使用钴或钨等重金属作为X射线管中的阳极材料[235]。在電子顯微鏡中,金、鉛、鉑、銥或鈾等重金屬可用來製造導電塗層,用染色、负染色或Template:Le等方法來調整生物樣本的電子密度[236]。在核能科學中,有時使用鉻、鐵、鋅的原子核轰击目標重金屬原子核,以產生超重元素[237],有時也會用重金屬作為Template:Le的目標,以形成中子[238]或放射性同位素(像砈可以用鉛、鉍、釷或鈾來製備)[239]。 Template:Clear
参见
註釋
參考資料
引用
文献
- Template:AnchorAhrland S., Template:Tsl J. O. & Rydberg J. 1973, "Solution chemistry," in J. C. Bailar & Template:Tsl (eds), Comprehensive Inorganic Chemistry, vol. 5, The Actinides, Template:Tsl, Oxford.
- Template:AnchorAlbutt M. & Dell R. 1963, The nitrites and sulphides of uranium, thorium and plutonium: A review of present knowledge, UK Atomic Energy Authority Research Group, Template:Tsl, Berkshire.
- Template:AnchorAlves A. K., Berutti, F. A. & Sánche, F. A. L. 2012, "Nanomaterials and catalysis", in C. P. Bergmann & M. J. de Andrade (ads), Nanonstructured Materials for Engineering Applications, Springer-Verlag, Berlin, Template:ISBN.
- Template:AnchorAmasawa E., Yi Teah H., Yu Ting Khew, J., Ikeda I. & Onuki M. 2016, "Drawing Lessons from the Minamata Incident for the General Public: Exercise on Resilience, Minamata Unit AY2014", in M. Esteban, T. Akiyama, C. Chen, I. Ikea, T. Mino (eds), Sustainability Science: Field Methods and Exercises, Springer International, Switzerland, pp. 93–116, Template:Doi Template:ISBN.
- Template:AnchorAriel E., Barta J. & Brandon D. 1973, "Preparation and properties of heavy metals", Powder Metallurgy International, vol. 5, no. 3, pp. 126–129.
- Template:AnchorAtlas R. M. 1986, Basic and Practical Microbiology, Macmillan Publishing Company, New York, Template:ISBN.
- Template:AnchorAustralian Government 2016, National Pollutant Inventory Template:Wayback, Department of the Environment and Energy, accessed 16 August 2016.
- Template:AnchorBaird C. & Cann M. 2012, Environmental Chemistry, 5th ed., Template:Tsl, New York, Template:ISBN.
- Template:AnchorBaldwin D. R. & Marshall W. J. 1999, "Heavy metal poisoning and its laboratory investigation", Template:Tsl, vol. 36, no. 3, pp. 267–300, Template:DOI.
- Template:AnchorBall J. L., Moore A. D. & Turner S. 2008, Ball and Moore's Essential Physics for Radiographers, 4th ed., Template:Tsl, Chichester, Template:ISBN.
- Template:AnchorBánfalvi G. 2011, "Heavy metals, trace elements and their cellular effects", in G. Bánfalvi (ed.), Cellular Effects of Heavy Metals, Springer, Dordrecht, pp. 3–28, Template:ISBN.
- Template:AnchorBaranoff E. 2015, "First-row transition metal complexes for the conversion of light into electricity and electricity into light", in W-Y Wong (ed.), Organometallics and Related Molecules for Energy Conversion, Springer, Heidelberg, pp. 61–90, Template:ISBN.
- Template:AnchorBerea E., Rodriguez-lbelo M. & Navarro J. A. R. 2016, "Platinum Group Metal—Organic frameworks" in S. Kaskel (ed.), The Chemistry of Metal-Organic Frameworks: Synthesis, Characterisation, and Applications, vol. 2, Wiley-VCH Weinheim, pp. 203–230, Template:ISBN.
- Template:AnchorBerger A. J. & Bruning N. 1979, Lady Luck's Companion: How to Play ... How to Enjoy ... How to Bet ... How to Win, Harper & Row, New York, Template:ISBN.
- Template:AnchorBerry L. G. & Mason B. 1959, Mineralogy: Concepts, Descriptions, Determinations, W. H. Freeman and Company, San Francisco.
- Template:AnchorBiddle H. C. & Bush G. L 1949, Chemistry Today, Rand McNally, Chicago.
- Template:AnchorBonchev D. & Kamenska V. 1981, "Predicting the properties of the 113–120 transactinide elements", The Journal of Physical Chemistry, vo. 85, no. 9, pp. 1177–1186, Template:DOI.
- Template:AnchorBonetti A., Leone R., Muggia F. & Howell S. B. (eds) 2009, Platinum and Other Heavy Metal Compounds in Cancer Chemotherapy: Molecular Mechanisms and Clinical Applications, Template:Tsl Press, New York, Template:ISBN.
- Template:AnchorBooth H. S. 1957, Inorganic Syntheses, vol. 5, McGraw-Hill, New York.
- Template:AnchorBradl H. E. 2005, "Sources and origins of heavy metals", in Bradl H. E. (ed.), Heavy Metals in the Environment: Origin, Interaction and Remediation, Elsevier, Amsterdam, Template:ISBN.
- Template:AnchorBrady J. E. & Holum J. R. 1995, Chemistry: The Study of Matter and its Changes, 2nd ed., John Wiley & Sons, New York, Template:ISBN.
- Template:AnchorBrephohl E. & Template:Tsl (ed) 2001, The Theory and Practice of Goldsmithing, C. Lewton-Brain trans., Brynmorgen Press, Portland, Maine, Template:ISBN.
- Template:AnchorBrown I. 1987, "Astatine: Its organonuclear chemistry and biomedical applications," in H. J. Emeléus & A. G. Sharpe (eds), Advances in Inorganic Chemistry, vol. 31, Template:Tsl, Orlando, pp. 43–88, Template:ISBN.
- Template:AnchorBryson R. M. & Hammond C. 2005, "Generic methodologies for nanotechnology: Characterisation"', in R. Kelsall, I. W. Hamley & M. Geoghegan, Nanoscale Science and Technology, John Wiley & Sons, Chichester, pp. 56–129, Template:ISBN.
- Template:AnchorBurkett B. 2010, Sport Mechanics for Coaches, 3rd ed., Human Kinetics, Champaign, Illinois, Template:ISBN.
- Template:AnchorCasey C. 1993, "Restructuring work: New work and new workers in post-industrial production", in R. P. Coulter & I. F. Goodson (eds), Rethinking Vocationalism: Whose Work/life is it?, Our Schools/Our Selves Education Foundation, Toronto, Template:ISBN.
- Template:AnchorChakhmouradian A.R., Smith M. P. & Kynicky J. 2015, "From "strategic" tungsten to "green" neodymium: A century of critical metals at a glance", Ore Geology Reviews, vol. 64, January, pp. 455–458, Template:DOI.
- Template:AnchorTemplate:Tsl 1743, "Metal Template:Wayback", in Cyclopedia: Or an Universal Dictionary of Arts and Sciences (etc.), vol. 2, D. Midwinter, London.
- Template:AnchorChandler D. E. & Roberson R. W. 2009, Bioimaging: Current Concepts in Light & Electron Microscopy, Template:Tsl, Boston, Template:ISBN.
- Template:AnchorChawla N. & Chawla K. K. 2013, Metal matrix composites, 2nd ed., Springer Science+Business Media, New York, Template:ISBN.
- Template:AnchorChen J. & Huang K. 2006, "A new technique for extraction of platinum group metals by pressure cyanidation", Hydrometallurgy, vol. 82, nos. 3–4, pp. 164–171, Template:DOI.
- Template:AnchorChoptuik M. W., Lehner L. & Pretorias F. 2015, "Probing strong-field gravity through numerical simulation", in A. Ashtekar, B. K. Berger, J. Isenberg & M. MacCallum (eds), General Relativity and Gravitation: A Centennial Perspective, Cambridge University Press, Cambridge, Template:ISBN.
- Template:AnchorTemplate:Tsl 2014, "Osmium tetroxide Template:Wayback", Template:Tsl, accessed 2 September 2016.
- Template:AnchorClose F. 2015, Nuclear Physics: A Very Short Introduction, Oxford University Press, Oxford, Template:ISBN.
- Template:AnchorClugston M & Flemming R 2000, Advanced Chemistry, Oxford University, Oxford, Template:ISBN.
- Template:AnchorCole M., Lindeque P., Halsband C. & Galloway T. S. 2011, "Microplastics as contaminants in the marine environment: A review", Marine Pollution Bulletin, vol. 62, no. 12, pp. 2588–2597, Template:DOI.
- Template:AnchorCole S. E. & Stuart K. R. 2000, "Nuclear and cortical histology for Template:Tsl", in D. J. Asai & J. D. Forney (eds), Methods in Cell Biology, vol. 62, Academic Press, San Diego, pp. 313–322, Template:ISBN.
- Template:AnchorCotton S. A. 1997, Chemistry of Precious Metals, Blackie Academic & Professional, London, Template:ISBN.
- Template:AnchorCotton S. 2006, Lanthanide and Actinide Chemistry, reprinted with corrections 2007, John Wiley & Sons, Chichester, Template:ISBN.
- Template:AnchorCox P. A. 1997, The elements: Their Origin, Abundance and Distribution, Oxford University Press, Oxford, Template:ISBN.
- Template:AnchorCrundwell F. K., Moats M. S., Ramachandran V., Robinson T. G. & Davenport W. G. 2011, Extractive Metallurgy of Nickel, Cobalt and Platinum Group Metals, Elsevier, Kidlington, Oxford, Template:ISBN.
- Template:AnchorCui X-Y., Li S-W., Zhang S-J., Fan Y-Y., Ma L. Q. 2015, "Toxic metals in children's toys and jewelry: Coupling bioaccessibility with risk assessment", Template:Tsl, vol. 200, pp. 77–84, Template:DOI.
- Template:AnchorDapena J. & Teves M. A. 1982, "Influence of the diameter of the hammer head on the distance of a hammer throw", Research Quarterly for Exercise and Sport, vol. 53, no. 1, pp. 78–81, Template:DOI.
- Template:AnchorDe Zuane J. 1997, Handbook of Drinking Water Quality, 2nd ed., John Wiley & Sons, New York, Template:ISBN.
- Template:AnchorDepartment of the Navy 2009, Gulf of Alaska Navy Training Activities: Draft Environmental Impact Statement/Overseas Environmental Impact Statement Template:Wayback, U.S. Government, accessed 21 August 2016.
- Template:AnchorDeschlag J. O. 2011, "Nuclear fission", in A. Vértes, S. Nagy, Z. Klencsár, R. G. Lovas, F. Rösch (eds), Handbook of Nuclear Chemistry, 2nd ed., Springer Science+Business Media, Dordrecht, pp. 223–280, Template:ISBN.
- Template:AnchorDesoize B. 2004, "Metals and metal compounds in cancer treatment", Template:Tsl, vol. 24, no. 3a, pp. 1529–1544, Template:Pmid.
- Template:AnchorDev N. 2008, 'Modelling Selenium Fate and Transport in Great Salt Lake Wetlands', PhD dissertation, University of Utah, ProQuest, Ann Arbor, Michigan, Template:ISBN.
- Template:AnchorTemplate:Tsl 2001, Forensic Pathology, 2nd ed., CRC Press, Boca Raton, Template:ISBN.
- Template:Anchor{Di Maio V. J. M. 2016, Gunshot Wounds: Practical Aspects of Firearms, Ballistics, and Forensic Techniques, 3rd ed., Template:Tsl, Boca Raton, Florida, Template:ISBN.
- Template:AnchorDuffus J. H. Template:Wayback 2002, " 'Heavy metals'—A meaningless term?" Template:Wayback, Pure and Applied Chemistry, vol. 74, no. 5, pp. 793–807, Template:DOI.
- Template:AnchorDunn P. 2009, Unusual metals could forge new cancer drugs Template:Wayback, University of Warwick, accessed 23 March 2016.
- Template:AnchorEbbing D. D. & Gammon S. D. 2017, General Chemistry, 11th ed., Cengage Learning, Boston, Template:ISBN.
- Template:AnchorEdelstein N. M., Fuger J., Katz J. L. & Morss L. R. 2010, "Summary and comparison of properties of the actinde and transactinide elements," in L. R. Morss, N. M. Edelstein & J. Fuger (eds), The Chemistry of the Actinide and Transactinide Elements, 4th ed., vol. 1–6, Template:Tsl, Dordrecht, pp. 1753–1835, Template:ISBN.
- Template:AnchorEisler R. 1993, Zinc Hazards to Fish, Wildlife, and Invertebrates: A Synoptic Review Template:Wayback, Biological Report 10, U. S. Department of the Interior, Laurel, Maryland, accessed 2 September 2016.
- Template:AnchorElliott S. B. 1946, The Alkaline-earth and Heavy-metal Soaps, Reinhold Publishing Corporation, New York.
- Template:AnchorEmsley J. 2011, Nature's Building Blocks: An A-Z Guide to the Elements Template:Wayback, new edition, Oxford University Press, Oxford, Template:ISBN.
- Template:AnchorEverts S. 2016, "What chemicals are in your tattoo Template:Wayback", Chemical & Engineering News, vol. 94, no. 33, pp. 24–26.
- Template:AnchorFournier J. 1976, "Bonding and the electronic structure of the actinide metals," Template:Tsl, vol 37, no. 2, pp. 235–244, Template:DOI.
- Template:AnchorFrick J. P. (ed.) 2000, Woldman's Engineering Alloys, 9th ed., ASM International, Materials Park, Ohio, Template:ISBN.
- Template:AnchorFrommer H. H. & Stabulas-Savage J. J. 2014, Radiology for the Dental Professional, 9th ed., Template:Tsl, St. Louis, Missouri, Template:ISBN.
- Template:AnchorGidding J. C. 1973, Chemistry, Man, and Environmental Change: An Integrated Approach, Canfield Press, New York, Template:ISBN.
- Template:AnchorGmelin L. 1849, Hand-book of chemistry, vol. III, Metals, translated from the German by H. Watts, Cavendish Society, London.
- Template:AnchorGoldsmith R. H. 1982, "Metalloids", Template:Tsl, vol. 59, no. 6, pp. 526–527, Template:DOI.
- Template:AnchorGorbachev V. M., Zamyatnin Y. S. & Lbov A. A. 1980, Nuclear Reactions in Heavy Elements: A Data Handbook, Pergamon Press, Oxford, Template:ISBN.
- Template:AnchorTemplate:Tsl & Headrick D. 2003, A Dictionary of Entomology, CABI Publishing, Wallingford, Template:ISBN.
- Template:AnchorGreenberg B. R. & Patterson D. 2008, Art in Chemistry; Chemistry in Art, 2nd ed., Teachers Ideas Press, Westport, Connecticut, Template:ISBN.
- Template:AnchorGribbon J. 2016, 13.8: The Quest to Find the True Age of the Universe and the Theory of Everything, Yale University Press, New Haven, Template:ISBN.
- Template:AnchorGschneidner Jr., K. A. 1975, Inorganic compounds, in C. T. Horowitz (ed.), Scandium: Its Occurrence, Chemistry, Physics, Metallurgy, Biology and Technology, Academic Press, London, pp. 152–251, Template:ISBN.
- Template:AnchorGuandalini G. S., Zhang L., Fornero E., Centeno J. A., Mokashi V. P., Ortiz P. A., Stockelman M. D., Osterburg A. R. & Chapman G. G. 2011, "Tissue distribution of tungsten in mice following oral exposure to sodium tungstate," Template:Tsl, vol. 24, no. 4, pp 488–493, Template:DOI.
- Template:AnchorGuney M. & Zagury G. J. 2012, "Heavy metals in toys and low-cost jewelry: Critical review of U.S. and Canadian legislations and recommendations for testing", Template:Tsl, vol. 48, pp. 1238–1246, Template:DOI.
- Template:AnchorHabashi F. 2009, "Gmelin and his Handbuch" Template:Wayback, Template:Tsl, vol. 34, no. 1, pp. 30–1.
- Template:AnchorHadhazy A. 2016, "Galactic 'gold mine' explains the origin of nature's heaviest elements Template:Wayback", Science Spotlights, 10 May 2016, accessed 11 July 2016.
- Template:AnchorHartmann W. K. 2005, Moons & Planets, 5th ed., Template:Tsl, Belmont, California, Template:ISBN.
- Template:AnchorHarvey P. J., Handley H. K. & Taylor M. P. 2015, "Identification of the sources of metal (lead) contamination in drinking waters in north-eastern Tasmania using lead isotopic compositions," Environmental Science and Pollution Research, vol. 22, no. 16, pp. 12276–12288, Template:DOI Template:PMID.
- Template:AnchorHasan S. E. 1996, Geology and Hazardous Waste Management, Prentice Hall, Upper Saddle River, New Jersey, Template:ISBN.
- Template:AnchorHawkes S. J. 1997, "What is a "heavy metal"?", Journal of Chemical Education, vol. 74, no. 11, p. 1374, Template:DOI.
- Template:AnchorHaynes W. M. 2015, CRC Handbook of Chemistry and Physics, 96th ed., CRC Press, Boca Raton, Florida, Template:ISBN.
- Template:AnchorHendrickson D. J. 2916, "Effects of early experience on brain and body", in D. Alicata, N. N. Jacobs, A. Guerrero and M. Piasecki (eds), Problem-based Behavioural Science and Psychiatry 2nd ed., Springer, Cham, pp. 33–54, Template:ISBN.
- Template:AnchorHermann A., Hoffmann R. & Neil Ashcroft 2013, "Condensed astatine: Monatomic and metallic Template:Wayback", Physical Review Letters, vol. 111, pp. 11604–1−11604-5, Template:DOI.
- Template:AnchorHerron N. 2000, "Cadmium compounds," in Kirk-Othmer Encyclopedia of Chemical Technology, vol. 4, John Wiley & Sons, New York, pp. 507–523, Template:ISBN.
- Template:AnchorHoffman D. C., Lee D. M. & Pershina V. 2011, "Transactinide elements and future elements," in L. R. Morss, N. Edelstein, J. Fuger & J. J. Katz (eds), The Chemistry of the Actinide and Transactinide Elements, 4th ed., vol. 3, Springer, Dordrecht, pp. 1652–1752, Template:ISBN.
- Template:AnchorHofmann S. 2002, On Beyond Uranium: Journey to the End of the Periodic Table, Taylor & Francis, London, Template:ISBN.
- Template:AnchorHousecroft J. E. 2008, Inorganic Chemistry, Elsevier, Burlington, Massachusetts, Template:ISBN.
- Template:AnchorHowell N., Lavers J., Paterson D., Garrett R. & Banati R. 2012, Trace metal distribution in feathers from migratory, pelagic birds Template:Wayback, Template:Tsl, accessed 3 May 2014.
- Template:AnchorHübner R., Astin K. B. & Herbert R. J. H. 2010, " 'Heavy metal'—time to move on from semantics to pragmatics?", Template:Tsl, vol. 12, pp. 1511–1514, Template:DOI.
- Template:AnchorIkehata K., Jin Y., Maleky N. & Lin A. 2015, "Heavy metal pollution in water resources in China—Occurrence and public health implications", in S. K. Sharma (ed.), Heavy Metals in Water: Presence, Removal and Safety, Royal Society of Chemistry, Cambridge, pp. 141–167, Template:ISBN.
- Template:AnchorInternational Antimony Association 2016, Antimony compounds Template:Wayback, accessed 2 September 2016.
- Template:AnchorInternational Platinum Group Metals Association n.d., The Primary Production of Platinum Group Metals (PGMs) Template:Wayback, accessed 4 September 2016.
- Template:AnchorIsmail A. F., Khulbe K. & Matsuura T. 2015, Gas Separation Membranes: Polymeric and Inorganic, Springer, Cham, Switzerland, Template:ISBN.
- Template:AnchorIUPAC 2016, "IUPAC is naming the four new elements nihonium, moscovium, tennessine, and oganesson Template:Wayback" accessed 27 August 2016.
- Template:AnchorIyengar G. V. 1998, "Reevaluation of the trace element content in Reference Man", Radiation Physics and Chemistry, vol. 51, nos 4–6, pp. 545–560, Template:Doi
- Template:AnchorJackson J. & Summitt J. 2006, The Modern Guide to Golf Clubmaking: The Principles and Techniques of Component Golf Club Assembly and Alteration, 5th ed., Hireko Trading Company, City of Industry, California, Template:ISBN.
- Template:AnchorJärup L 2003, "Hazards of heavy metal contamination", Template:Tsl, vol. 68, no. 1, pp. 167–182, Template:DOI.
- Template:AnchorJones C. J. 2001, d- and f-Block Chemistry, Royal Society of Chemistry, Cambridge, Template:ISBN.
- Template:AnchorKantra S. 2001, "What's new", Popular Science, vol. 254, no. 4, April, p. 10.
- Template:AnchorKeller C., Wolf W. & Shani J. 2012, "Radionuclides, 2. Radioactive elements and artificial radionuclides", in F. Ullmann (ed.), Template:Tsl, vol. 31, Wiley-VCH, Weinheim, pp. 89–117, Template:DOI.
- Template:AnchorKing R. B. 1995, Inorganic Chemistry of Main Group Elements, Template:Tsl, New York, Template:ISBN.
- Template:AnchorTemplate:Tsl & Elving P. J. FR 1964, Treatise on Analytical Chemistry, part II, vol. 6, Interscience Encyclopedia, New York, Template:ISBN.
- Template:AnchorKorenman I. M. 1959, "Regularities in properties of thallium", Journal of General Chemistry of the USSR, English translation, Consultants Bureau, New York, vol. 29, no. 2, pp. 1366–90, Template:ISSN.
- Template:AnchorKozin L. F. & Hansen S. C. 2013, Mercury Handbook: Chemistry, Applications and Environmental Impact, RSC Publishing, Cambridge, Template:ISBN.
- Template:AnchorKumar R., Srivastava P. K., Srivastava S. P. 1994, "Leaching of heavy metals (Cr, Fe, and Ni) from stainless steel utensils in food simulates and food materials", Bulletin of Environmental Contamination and Toxicology, vol. 53, no. 2, Template:DOI, pp. 259–266.
- Template:AnchorLach K., Steer B., Gorbunov B., Mička V. & Muir R. B. 2015, "Evaluation of exposure to airborne heavy metals at gun shooting ranges", Template:Tsl, vol. 59, no. 3, pp. 307–323, Template:DOI.
- Template:AnchorLandis W., Sofield R. & Yu M-H. 2010, Introduction to Environmental Toxicology: Molecular Substructures to Ecological Landscapes, 4th ed., CRC Press, Boca Raton, Florida, Template:ISBN.
- Template:AnchorLane T. W., Saito M. A., George G. N., Pickering, I. J., Prince R. C. & Morel F. M. M. 2005, "Biochemistry: A cadmium enzyme from a marine diatom", Nature, vol. 435, no. 7038, p. 42, Template:DOI.
- Template:AnchorLee J. D. 1996, Concise Inorganic Chemistry, 5th ed., Template:Tsl, Oxford, Template:ISBN.
- Template:AnchorLeeper G. W. 1978, Managing the Heavy Metals on the Land Template:Tsl, New York, Template:ISBN.
- Template:AnchorLemony A. D. 1997, "A teratogenic deformity index for evaluating impacts of selenium on fish populations", Ecotoxicology and Environmental Safety, vol. 37, no. 3, pp. 259–266, Template:DOI.
- Template:AnchorLide D. R. (ed.) 2004, CRC Handbook of Chemistry and Physics, 85th ed., CRC Press, Boca Raton, Florida, Template:ISBN.
- Template:AnchorLiens J. 2010, "Heavy metals as pollutants", in B. Warf (ed.), Encyclopaedia of Geography, Sage Publications, Thousand Oaks, California, pp. 1415–1418, Template:ISBN.
- Template:AnchorLima E., Guerra R., Lara V. & Guzmán A. 2013, "Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi " Template:Tsl, vol. 7:11, Template:DOI Template:PMID Template:PMC.
- Template:AnchorLitasov K. D. & Shatskiy A. F. 2016, "Composition of the Earth's core: A review", Russian Geology and Geophysics, vol. 57, no. 1, pp. 22–46, Template:DOI.
- Template:AnchorLivesey A. 2012, Advanced Motorsport Engineering, Routledge, London, Template:ISBN.
- Template:AnchorLivingston R. A. 1991, "Influence of the Environment on the Patina of the Statue of Liberty", Environmental Science & Technology, vol. 25, no. 8, pp. 1400–1408, Template:DOI.
- Template:AnchorLongo F. R. 1974, General Chemistry: Interaction of Matter, Energy, and Man, Template:Tsl, New York, Template:ISBN.
- Template:AnchorLove M. 1998, Phasing Out Lead from Gasoline: Worldwide Experience and Policy Implications, World Bank Technical Paper volume 397, The World Bank, Washington DC, Template:ISBN.
- Template:AnchorLyman W. J. 1995, "Transport and transformation processes", in Fundamentals of Aquatic Toxicology, G. M. Rand (ed.), Taylor & Francis, London, pp. 449–492, Template:ISBN.
- Template:AnchorMacintyre J. E. 1994, Dictionary of inorganic compounds, supplement 2, Dictionary of Inorganic Compounds, vol. 7, Template:Tsl, London, Template:ISBN.
- Template:AnchorMacKay K. M., MacKay R. A. & Henderson W. 2002, Introduction to Modern Inorganic Chemistry, 6th ed., Nelson Thornes, Cheltenham, Template:ISBN.
- Template:AnchorMagee R. J. 1969, Steps to Atomic Power, Cheshire for La Trobe University, Melbourne.
- Template:AnchorMagill F. N. I (ed.) 1992, Magill's Survey of Science, Physical Science series, vol. 3, Salem Press, Pasadena, Template:ISBN.
- Template:AnchorMartin M. H. & Coughtrey P. J. 1982, Biological Monitoring of Heavy Metal Pollution, Applied Science Publishers, London, Template:ISBN.
- Template:AnchorMassarani M. 2015, "Brazilian mine disaster releases dangerous metals Template:Wayback," Chemistry World, November 2015, accessed 16 April 2016.
- Template:AnchorMasters C. 1981, Homogenous Transition-metal Catalysis: A Gentle Art, Chapman and Hall, London, Template:ISBN.
- Template:AnchorMatyi R. J. & Baboian R. 1986, "An X-ray Diffraction Analysis of the Patina of the Statue of Liberty", Powder Diffraction, vol. 1, no. 4, pp. 299–304, Template:DOI.
- Template:AnchorMcColm I. J. 1994, Dictionary of Ceramic Science and Engineering, 2nd ed., Springer Science+Business Media, New York, Template:ISBN.
- Template:AnchorMcCurdy R. M. 1975, Qualities and quantities: Preparation for College Chemistry, Template:Tsl, New York, Template:ISBN.
- Template:AnchorMcLemore V. T. (ed.) 2008, Basics of Metal Mining Influenced Water, vol. 1, Society for Mining, Metallurgy, and Exploration, Littleton, Colorado, Template:ISBN.
- Template:AnchorMcQueen K. G. 2009, Regolith geochemistry, in K. M. Scott & C. F. Pain (eds), Regolith Science, Template:Tsl, Collingwood, Victoria, Template:ISBN.
- Template:AnchorTemplate:Tsl 1924, A comprehensive Treatise on Inorganic and Theoretical Chemistry, vol. 5, Longmans, Green and Company, London.
- Template:AnchorMoore J. W. & Ramamoorthy S. 1984, Heavy Metals in Natural Waters: Applied Monitoring and Impact Assessment, Springer Verlag, New York, Template:ISBN.
- Template:AnchorMorris C. G. 1992, Academic Press Dictionary of Science and Technology, Harcourt Brace Jovanovich, San Diego, Template:ISBN.
- Template:AnchorMorstein J. H. 2005, "Fat Man", in E. A. Croddy & Y. Y. Wirtz (eds), Weapons of Mass Destruction: An Encyclopedia of Worldwide Policy, Technology, and History, Template:Tsl, Santa Barbara, California, Template:ISBN.
- Template:AnchorMoselle B. (ed.) 2005, 2004 National Home Improvement Estimator, Craftsman Book Company, Carlsbad, California, Template:ISBN.
- Template:AnchorNaja G. M. & Volesky B. 2009, "Toxicity and sources of Pb, Cd, Hg, Cr, As, and radionuclides", in L. K. Wang, J. P. Chen, Y. Hung & N. K. Shammas, Heavy Metals in the Environment, CRC Press, Boca Raton, Florida, Template:ISBN.
- Template:AnchorNakbanpote W., Meesungneon O. & Prasad M. N. V. 2016, "Potential of ornamental plants for phytoremediation of heavy metals and income generation", in M. N. V. Prasad (ed.), Bioremediation and Bioeconomy, Elsevier, Amsterdam, pp. 179–218, Template:ISBN.
- Template:AnchorNathans M. W. 1963, Elementary Chemistry, Prentice Hall, Englewood Cliffs, New Jersey.
- Template:AnchorNational Materials Advisory Board 1971, Trends in the Use of Depleted Uranium, National Academy of Sciences – National Academy of Engineering, Washington DC.
- Template:AnchorNational Materials Advisory Board 1973, Trends in Usage of Tungsten, National Academy of Sciences – National Academy of Engineering, Washington DC.
- Template:AnchorNational Organization for Rare Disorders 2015, Heavy metal poisoning Template:Wayback, accessed 3 March 2016.
- Template:AnchorNatural Resources Canada 2015, "Generation of the Earth's magnetic field Template:Wayback", accessed 30 August 2016.
- Template:AnchorNieboer E. & Richardson D. 1978, "Lichens and 'heavy metals' ", International Lichenology Newsletter, vol. 11, no. 1, pp. 1–3.
- Template:AnchorNieboer E. & Richardson D. H. S. 1980, "The replacement of the nondescript term 'heavy metals' by a biologically and chemically significant classification of metal ions", Environmental Pollution Series B, Chemical and Physical, vol. 1, no. 1, pp. 3–26, Template:DOI.
- Template:AnchorNzierżanowski K. & Gawroński S. W. 2012, "Heavy metal concentration in plants growing on the vicinity of railroad tracks: a pilot study Template:Wayback", Challenges of Modern Technology, vol. 3, no. 1, pp. 42–45, Template:ISSN, accessed 21 August 2016.
- Template:AnchorOhlendorf H. M. 2003, "Ecotoxicology of selenium", in D. J. Hoffman, B. A. Rattner, G. A. Burton & Template:Tsl, Handbook of Ecotoxicology, 2nd ed., Template:Tsl, Boca Raton, pp. 466–491, Template:ISBN.
- Template:AnchorOndreička R., Kortus J. & Ginter E. 1971, "Aluminium, its absorption, distribution, and effects on phosphorus metabolism", in S. C. Skoryna & D. Waldron-Edward (eds), Intestinal Absorption of Metal Ions, Trace Elements and Radionuclides, Pergamon press, Oxford.
- Template:AnchorOng K. L., Tan T. H. & Cheung W. L. 1997, "Potassium permanganate poisoning—a rare cause of fatal poisoning", Journal of Accident & Emergency Medicine, vol. 14, no. 1, pp. 43–45, Template:PMC.
- Template:AnchorOxford English Dictionary 1989, 2nd ed., Oxford University Press, Oxford, Template:ISBN.
- Template:AnchorPacheco-Torgal F., Jalali S. & Fucic A. (eds) 2012, Toxicity of building materials, Template:Tsl, Oxford, Template:ISBN.
- Template:AnchorTemplate:Tsl 2001, Theoretical Astrophysics, vol. 2, Stars and Stellar Systems, Cambridge University Press, Cambridge, Template:ISBN.
- Template:AnchorPan W. & Dai J. 2015, "ADS based on linear accelerators", in W. Chao & W. Chou (eds), Reviews of accelerator science and technology, vol. 8, Accelerator Applications in Energy and Security, Template:Tsl, Singapore, pp. 55–76, Template:ISBN.
- Template:AnchorParish R. V. 1977, The Metallic Elements, Longman, New York, Template:ISBN.
- Template:AnchorPerry J. & Vanderklein E. L. Water Quality: Management of a Natural Resource, Blackwell Science, Cambridge, Massachusetts Template:ISBN.
- Template:AnchorPickering N. C. 1991, The Bowed String: Observations on the Design, Manufacture, Testing and Performance of Strings for Violins, Violas and Cellos, Amereon, Mattituck, New York.
- Template:AnchorPodosek F. A. 2011, "Noble gases", in H. D. Holland & Template:Tsl (eds), Isotope Geochemistry: From the Treatise on Geochemistry, Elsevier, Amsterdam, pp. 467–492, Template:ISBN.
- Template:AnchorPodsiki C. 2008, "Heavy metals, their salts, and other compounds Template:Wayback", Template:Tsl, November, special insert, pp. 1–4.
- Template:AnchorPreschel J. July 29, 2005, "Green bullets not so eco-friendly Template:Wayback", CBS News, accessed 18 March 2016.
- Template:AnchorPreuss P. 17 July 2011, "What keeps the Earth cooking? Template:Wayback," Berkeley Lab, accessed 17 July 2016.
- Template:AnchorTemplate:Tsl 2011, The Adventures of a Cello: Revised Edition, with a New Epilogue, Template:Tsl, Austin, Template:ISBN
- Template:AnchorRaghuram P., Soma Raju I. V. & Sriramulu J. 2010, "Heavy metals testing in active pharmaceutical ingredients: an alternate approach", Template:Tsl, vol. 65, no. 1, pp. 15–18, Template:DOI.
- Template:AnchorRainbow P. S. 1991, "The biology of heavy metals in the sea", in J. Rose (ed.), Water and the Environment, Template:Tsl, Philadelphia, pp. 415–432, Template:ISBN.
- Template:AnchorRand G. M., Wells P. G. & McCarty L. S. 1995, "Introduction to aquatic toxicology", in G. M. Rand (ed.), Fundamentals of Aquatic Toxicology: Effects, Environmental Fate and Risk Assessment, 2nd ed., Taylor & Francis, London, pp. 3–70, Template:ISBN.
- Template:AnchorRankin W. J. 2011, Minerals, Metals and Sustainability: Meeting Future Material Needs, CSIRO Publishing, Collingwood, Victoria, Template:ISBN.
- Template:AnchorRasic-Milutinovic Z. & Jovanovic D. 2013, "Toxic metals", in M. Ferrante, G. Oliveri Conti, Z. Rasic-Milutinovic & D. Jovanovic (eds), Health Effects of Metals and Related Substances in Drinking Water, IWA Publishing, London, Template:ISBN.
- Template:AnchorRaymond R. 1984, Out of the Fiery Furnace: The Impact of Metals on the History of Mankind, Macmillan, South Melbourne, Template:ISBN.
- Template:AnchorRebhandl W., Milassin A., Brunner L., Steffan I., Benkö T., Hörmann M., Burschen J. 2007, "In vitro study of ingested coins: Leave them or retrieve them?", Journal of Paediatric Surgery, vol. 42, no. 10, pp. 1729–1734, Template:DOI.
- Template:AnchorRehder D. 2010, Chemistry in Space: From Interstellar Matter to the Origin of Life, Wiley-VCH, Weinheim, Template:ISBN.
- Template:AnchorRenner H., Schlamp G., Kleinwächter I., Drost E., Lüchow H. M., Tews P., Panster P., Diehl M., Lang J., Kreuzer T., Knödler A., Starz K. A., Dermann K., Rothaut J., Drieselmann R., Peter C. & Schiele R. 2012, "Platinum Group Metals and compounds", in F. Ullmann (ed.), Ullmann's Encyclopedia of Industrial Chemistry, vol. 28, Wiley-VCH, Weinheim, pp. 317–388, Template:DOI.
- Template:AnchorReyes J. W. 2007, Environmental Policy as Social Policy? The Impact of Childhood Lead Exposure on Crime Template:Wayback, National Bureau of Economic Research Working Paper 13097, accessed 16 October 2016.
- Template:AnchorTemplate:Tsl (ed.) 2012, Oxford Dictionary of Astronomy, 2nd ed. rev., Oxford University Press, New York, Template:ISBN.
- Template:AnchorRockhoff H. 2012, America's Economic Way of War: War and the US Economy from the Spanish–American War to the Persian Gulf War, Cambridge University Press, Cambridge, Template:ISBN.
- Template:AnchorRoe J. & Roe M. 1992, "World's coinage uses 24 chemical elements", World Coinage News, vol. 19, no. 4, pp. 24–25; no. 5, pp. 18–19.
- Template:AnchorRussell A. M. & Lee K. L. 2005, Structure–Property Relations in Nonferrous Metals, John Wiley & Sons, Hoboken, New Jersey, Template:ISBN.
- Template:AnchorRusyniak D. E., Arroyo A., Acciani J., Froberg B., Kao L. & Furbee B. 2010, "Heavy metal poisoning: Management of intoxication and antidotes", in A. Luch (ed.), Molecular, Clinical and Environmental Toxicology, vol. 2, Birkhäuser Verlag, Basel, pp. 365–396, Template:ISBN.
- Template:AnchorRyan J. 2012, Personal Financial Literacy, 2nd ed., South-Western, Mason, Ohio, Template:ISBN.
- Template:AnchorSamsonov G. V. (ed.) 1968, Handbook of the Physicochemical Properties of the Elements, IFI-Plenum, New York, Template:ISBN.
- Template:AnchorSanders R. 2003, "Radioactive potassium may be major heat source in Earth's core Template:Wayback," UCBerkelyNews, 10 December, accessed 17 July 20016.
- Template:AnchorSchweitzer P. A. 2003, Metallic materials: Physical, Mechanical, and Corrosion properties, Marcel Dekker, New York, Template:ISBN.
- Template:AnchorTemplate:Tsl & Pesterfield L. L. 2010, The Aqueous Chemistry of the Elements, Oxford University Press, Oxford, Template:ISBN.
- Template:AnchorScott R. M. 1989, Chemical Hazards in the Workplace, CRC Press, Boca Raton, Orlando, Template:ISBN.
- Template:AnchorScoullos M. (ed.), Vonkeman G. H., Thornton I. & Makuch Z. 2001, Mercury — Cadmium — Lead Handbook for Sustainable Heavy Metals Policy and Regulation, Kluwer Academic Publishers, Dordrecht, Template:ISBN.
- Template:AnchorSelinger B. 1978, Chemistry in the Market Place, 2nd ed., Australian National University Press, Canberra, Template:ISBN.
- Template:AnchorSeymour R. J. & O'Farrelly J. 2012, "Platinum Group Metals", Kirk-Other Encyclopaedia of Chemical Technology, John Wiley & Sons, New York, Template:DOI.
- Template:AnchorShaw B. P., Sahu S. K. & Mishra R. K. 1999, "Heavy metal induced oxidative damage in terrestrial plants", in M. N. V. Prased (ed.), Heavy Metal Stress in Plants: From Biomolecules to Ecosystems Springer-Verlag, Berlin, Template:ISBN.
- Template:AnchorShedd K. B. 2002, "Tungsten" Template:Wayback, Minerals Yearbook, United States Geological Survey.
- Template:AnchorTemplate:Tsl 1950, The Chemical Elements and their Compounds, vol. 1, Oxford University Press, London.
- Template:AnchorSilva R. J. 2010, "Fermium, mendelevium, nobelium, and lawrencium", in L. R. Morss, N. Edelstein & J. Fuger (eds), The Chemistry of the Actinide and Transactinide Elements, vol. 3, 4th ed., Springer, Dordrecht, pp. 1621–1651, Template:ISBN.
- Template:AnchorSpolek G. 2007, "Design and materials in fly fishing", in A. Subic (ed.), Materials in Sports Equipment, Volume 2, Woodhead Publishing, Abington, Cambridge, pp. 225–247, Template:ISBN.
- Template:AnchorStankovic S. & Stankocic A. R. 2013, "Bioindicators of toxic metals", in E. Lichtfouse, J. Schwarzbauer, D. Robert 2013, Green materials for energy, products and depollution, Springer, Dordrecht, Template:ISBN, pp. 151–228.
- Template:AnchorState Water Control Resources Board 1987, Toxic substances monitoring program, issue 79, part 20 of the Water Quality Monitoring Report, Sacramento, California.
- Template:AnchorTechnical Publications 1953, Fire Engineering, vol. 111, p. 235, Template:ISSN.
- Template:AnchorTemplate:Tsl, Light Metals Division 2016, accessed 22 June 2016.
- Template:AnchorThe United States Pharmacopeia 1985, 21st revision, The United States Pharmacopeial Convention, Rockville, Maryland, Template:ISBN.
- Template:AnchorThorne P. C. L. & Roberts E. R. 1943, Fritz Ephraim Inorganic Chemistry, 4th ed., Gurney and Jackson, London.
- Template:AnchorTisza M. 2001, Physical Metallurgy for Engineers, ASM International, Materials Park, Ohio, Template:ISBN.
- Template:AnchorTokar E. J., Boyd W. A., Freedman J. H. & Wales M. P. 2013, "Toxic effects of metals Template:Wayback", in C. D. Klaassen (ed.), Casarett and Doull's Toxicology: the Basic Science of Poisons, 8th ed., McGraw-Hill Medical, New York, Template:ISBN, accessed 9 September 2016 Template:Subscription required.
- Template:AnchorTomasik P. & Ratajewicz Z. 1985, Pyridine metal complexes, vol. 14, no. 6A, The Chemistry of Heterocyclic Compounds, John Wiley & Sons, New York, Template:ISBN.
- Template:AnchorTopp N. E. 1965, The Chemistry of the Rare-earth Elements, Elsevier Publishing Company, Amsterdam.
- Template:AnchorTorrice M. 2016, "How lead ended up in Flint's tap water Template:Wayback," Chemical & Engineering News, vol. 94, no. 7, pp. 26–27.
- Template:AnchorTretkoff E. 2006, "March 20, 1800: Volta describes the Electric Battery Template:Wayback", APS News, This Month in Physics History, American Physical Society, accessed 26 August 2016.
- Template:AnchorUden P. C. 2005, 'Speciation of Selenium,' in R. Cornelis, J. Caruso, H. Crews & K. Heumann (eds), Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine and Occupational Health, John Wiley & Sons, Chichester, pp. 346–65, Template:ISBN.
- Template:AnchorUnited States Environmental Protection Agency 1988, Ambient Aquatic Life Water Quality Criteria for Antimony (III), draft, Office of Research and Development, Environmental Research Laboratories, Washington.
- Template:AnchorUnited States Environmental Protection Agency 2014, Technical Fact Sheet–Tungsten Template:Wayback, accessed 27 March 2016.
- Template:AnchorUnited States Government 2014, Toxic Pollutant List Template:Wayback, Code of Federal Regulations, 40 CFR 401.15., accessed 27 March 2016.
- Template:AnchorValkovic V. 1990, "Origin of trace element requirements by living matter", in B. Gruber & J. H. Yopp (eds), Symmetries in Science IV: Biological and biophysical systems, Plenum Press, New York, pp. 213–242, Template:ISBN.
- Template:AnchorVanGelder K. T. 2014, Fundamentals of Automotive Technology: Principles and Practice, Template:Tsl, Burlington MA, Template:ISBN.
- Template:AnchorVenner M., Lessening M., Pankani D. & Strecker E. 2004, Identification of Research Needs Related to Highway Runoff Management Template:Wayback, Transportation Research Board, Washington DC, Template:ISBN, accessed 21 August 2016.
- Template:AnchorVenugopal B. & Luckey T. D. 1978, Metal Toxicity in Mammals, vol. 2, Plenum Press, New York, Template:ISBN.
- Template:AnchorVernon R. E. 2013, "Which elements are metalloids", Journal of Chemical Education, vol. 90, no. 12, pp. 1703–1707, Template:DOI.
- Template:AnchorVolesky B. 1990, Biosorption of Heavy Metals, CRC Press, Boca Raton, Template:ISBN.
- Template:Anchorvon Gleich A. 2013, "Outlines of a sustainable metals industry", in A. von Gleich, R. U. Ayres & S. Gößling-Reisemann (eds), Sustainable Metals Management, Springer, Dordrecht, pp. 3–40, Template:ISBN.
- Template:Anchorvon Zeerleder A. 1949, Technology of Light Metals, Elsevier Publishing Company, New York.
- Template:AnchorWarth A. H. 1956, The Chemistry and Technology of Waxes, Reinhold Publishing Corporation, New York.
- Template:AnchorTemplate:Tsl 1983, "The discovery of nuclear fission and a nuclear physics paradigm", in W. Shea (ed.), Otto Hahn and the Rise of Nuclear Physics, Template:Tsl Publishing Company, Dordrecht, pp. 91–133, Template:ISBN.
- Template:AnchorWeber D. J. & Rutula W. A. 2001, "Use of metals as microbicides in preventing infections in healthcare", in Disinfection, Sterilization, and Preservation, 5th ed., S. S. Block (ed.), Template:Tsl, Philadelphia, Template:ISBN.
- Template:AnchorWelter G. 1976, Cleaning and Preservation of Coins and Medals, S. J. Durst, New York, Template:ISBN.
- Template:AnchorWhite C. 2010, Projectile Dynamics in Sport: Principles and Applications, Routledge, London, Template:ISBN.
- Template:AnchorWiberg N. 2001, Inorganic Chemistry, Academic Press, San Diego, Template:ISBN.
- Template:AnchorWijayawardena M. A. A., Megharaj M. & Naidu R. 2016, "Exposure, toxicity, health impacts and bioavailability of heavy metal mixtures", in D. L. Sparks, Advances in Agronomy, vol. 138, pp. 175–234, Academic Press, London, Template:ISBN.
- Template:AnchorWingerson L. 1986, "America cleans up Liberty Template:Wayback", New Scientist, 25 December/1 January 1987, pp. 31–35, accessed 1 October 2016.
- Template:AnchorWong M. Y., Hedley G. J., Xie G., Kölln L. S, Samuel I. D. W., Pertegaś A., Bolink H. J., Mosman-Colman, E., "Light-emitting electrochemical cells and solution-processed organic light-emitting diodes using small molecule organic thermally activated delayed fluorescence emitters", Template:Tsl, vol. 27, no. 19, pp. 6535–6542, Template:DOI.
- Template:AnchorWulfsberg G. 1987, Principles of Descriptive Inorganic Chemistry, Brooks/Cole Publishing Company, Monterey, California, Template:ISBN.
- Template:AnchorWulfsberg G. 2000, Inorganic Chemistry, University Science Books, Sausalito, California, Template:ISBN.
- Template:AnchorYadav J. S., Antony A., Subba Reddy, B. V. 2012, "Bismuth(III) salts as synthetic tools in organic transformations", in T. Ollevier (ed.), Bismuth-mediated Organic Reactions, Topics in Current Chemistry 311, Springer, Heidelberg, Template:ISBN.
- Template:AnchorYang D. J., Jolly W. L. & O'Keefe A. 1977, "Conversion of hydrous germanium(II) oxide to germynyl sesquioxide, (HGe)2O3", Inorganic Chemistry, vol. 16, no. 11, pp. 2980–2982, Template:DOI.
- Template:AnchorYousif N. 2007, Geochemistry of stream sediment from the state of Colorado using NURE data, ETD Collection for the University of Texas, El Paso, paper AAI3273991 Template:Wayback.
- Template:AnchorTemplate:Cite journal
- Template:Anchor{cite journal |last1=Du |first1=Jian |last2=Chen |first2=Xin-liang |last3=Liu |first3=Cai-chi |last4=Ni |first4=Jian |last5=Hou |first5=Guo-fu |last6=Zhao |first6=Ying |last7=Zhang |first7=Xiao-dan |title=Highly transparent and conductive indium tin oxide thin films for solar cells grown by reactive thermal evaporation at low temperature |journal=Applied Physics A |date=2014-11 |volume=117 |issue=2 |pages=815–822 |doi=10.1007/s00339-014-8436-x |accessdate=2020-09-14}}
- Template:AnchorStwertka, Albert. A Guide to the Elements, Oxford University Press, 1996, p. 125. Template:ISBN
- Template:AnchorTemplate:Cite journal
- Template:AnchorTemplate:Cite journal
Template:化學元素分類 Template:Authority control
- ↑ John H. Duffus: "Heavy Metals"- A Meaningless Term, Chemistry International, November 2001, -{R|http://www.iupac.org/publications/ci/2001/november/heavymetals.html}- Template:Wayback
- ↑ 2.0 2.1 2.2 2.3 2.4 Template:Harvnb
- ↑ 3.0 3.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ 5.0 5.1 Template:Harvnb
- ↑ 6.0 6.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 9.0 9.1 9.2 9.3 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 15.0 15.1 Template:Harvnb}}
- ↑ Template:Harvnb
- ↑ 17.0 17.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb; Template:Harvnb; Template:Harvnb; Template:Harvnb
- ↑ 24.0 24.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 27.0 27.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 30.0 30.1 Template:Harvnb
- ↑ 31.0 31.1 31.2 Template:Harvnb
- ↑ Template:Harvnb
- ↑ 33.0 33.1 33.2 Template:Harvnb
- ↑ Template:Harvnb
- ↑ 35.0 35.1 Template:Harvnb
- ↑ 36.0 36.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb: "That which distinguishes metals from all other bodies ... is their heaviness ..."
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ 56.0 56.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 78.0 78.1 78.2 78.3 78.4 78.5 78.6 78.7 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 101.0 101.1 Template:Harvnb
- ↑ 102.0 102.1 102.2 Template:Harvnb
- ↑ Template:Harvnb
- ↑ 104.0 104.1 104.2 Template:Harvnb
- ↑ 105.0 105.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb; Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 140.0 140.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 143.0 143.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb. The authors note, however, that, "The sulfides of ... Ga(III) and Cr(III) tend to dissolve and/or decompose in water."
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 155.0 155.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ 引用错误:
<ref>标签无效;未给name(名称)为rsc的ref(参考)提供文本 - ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ 166.0 166.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 175.0 175.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ 190.0 190.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 226.0 226.1 Template:Harvnb}}
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 引用错误:
<ref>标签无效;未给name(名称)为tungsram的ref(参考)提供文本 - ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ 232.0 232.1 Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb; Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
- ↑ Template:Harvnb
引用错误:名称为“註”的group(分组)存在<ref>标签,但未找到对应的<references group="註"/>标签




