乙腈 的一些性质如下所述。
Template:Chembox header | 相性质
三相点 [ 3] 229.32 K (–43.83 °C), 167 Pa
临界点
545 K (272 °C), 4.87 MPa
标准熔化焓变 , Δfus H o 8.167 kJ/mol (crystal I → liq)
标准熔化熵变, Δfus S o
35.61 J/(mol·K) (crystal I → liq)
标准汽化焓变, Δvap H o
33.225 kJ/mol at 25°C
标准汽化熵变, Δvap S o
111.44 J/(mol·K) at 25°C
Template:Chembox header | 固体性质
标准摩尔生成焓 , Δf H o solid
? kJ/mol at 25°C
标准摩尔熵 ,S o solid
? J/(mol K)
热容量 , cp
92.36 J/(mol K)at 298.15 K
标准相变焓变, Δtrs H o
0.8979 kJ/mol at –56.2°C
标准相变熵变, Δtrs S o
4.14 J/(mol·K) at –56.2°
Template:Chembox header | 液体性质
标准摩尔生成焓 , Δf H o liquid
–40.56 kJ/mol
标准摩尔熵 ,S o liquid
149.62 J/(mol K)
燃烧热 , Δc H o –1256.33 kJ/mol
热容量 , cp
91.7 J/(mol K) at 25°C
Template:Chembox header | 气体性质
标准摩尔生成焓 , Δf H o gas
–74.04 kJ/mol
标准摩尔熵 ,S o gas
? J/(mol K)
燃烧热 , cp
? J/(mol K)
Table data obtained from CRC Handbook of Chemistry and Physics , 44th ed. The "(s)" notation indicates temperature of solid/vapor equilibrium. Otherwise the data is temperature of liquid/vapor equilibrium.
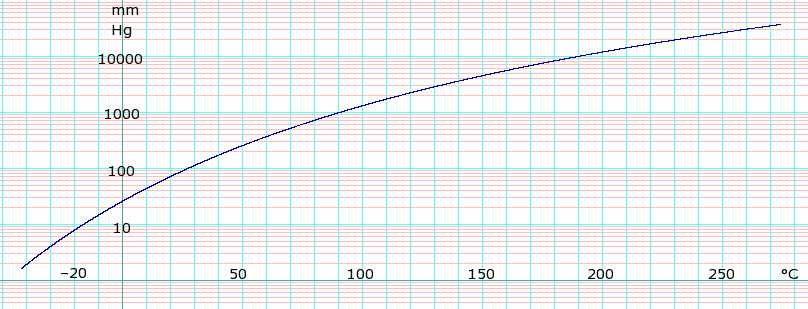
log10 of Acetonitrile vapor pressure. Uses formula log e P m m H g = log e ( 7 6 0 1 0 1 . 3 2 5 ) − 3 . 8 8 1 7 1 0 log e ( T + 2 7 3 . 1 5 ) − 4 9 9 9 . 6 1 8 T + 2 7 3 . 1 5 + 4 1 . 0 5 9 0 1 + 3 . 5 1 5 9 5 6 × 1 0 − 0 6 ( T + 2 7 3 . 1 5 ) 2 [ 2] Template:Clear
Vapor-liquid Equilibrium for Acetonitrile/Water [ 4] P = 760 mmHg
沸点(°C)
乙腈摩尔百分数
液相
气相
86.5
2.9
26.3
81.1
9.3
50.5
80.0
14.2
55.9
78.6
25.4
61.7
77.4
40.2
65.5
76.7
50.7
66.4
76.6
52.7
67.3
76.0
71.8
72.8
76.6
83.9
78.0
76.8
85.6
76.1
80.4
98.6
94.5
Vapor-liquid Equilibrium for Acetonitrile/Methanol [ 4] P = 760 mmHg
沸点(°C)
甲醇摩尔百分数
液相
气相
79.20
2.5
9.5
77.95
4.0
13.5
76.77
5.5
17.5
75.12
9.7
26.5
73.12
14.0
33.0
72.07
17.0
37.5
70.96
20.0
420
68.85
24.7
45.5
68.39
28.9
50.5
66.00
41.5
59.5
65.35
47.0
62.5
64.75
54.5
65.5
64.34
63.0
71.5
64.03
69.0
74.5
63.80
74.5
78.9
63.77
82.5
82.5
63.76
86.0
86.0
63.87
90.0
88.0
64.05
93.0
91.5
64.18
95.0
93.0
64.40
97.0
95.5
Vapor-liquid Equilibrium for Acetonitrile/Benzene [ 4] P = 760 mmHg
沸点(°C)
苯摩尔百分数
液相
气相
80.6
2.0
4.8
80.4
2.7
6.2
79.0
5.6
12.5
78.5
6.5
14.7
78.0
7.7
16.5
76.0
17.6
29.3
74.9
24.2
35.5
74.4
29.9
39.3
73.8
37.1
43.8
73.7
38.0
44.9
73.4
44.0
48.5
73.2
51.3
51.9
73.0
52.94
52.94
73.2
58.1
54.5
73.4
66.5
60.0
73.8
71.3
62.6
74.0
76.7
65.7
74.4
79.0
68.0
76.3
92.0
80.1
Vapor-liquid Equilibrium for Acetonitrile/Toluene [ 4] P = 760 mmHg
沸点(°C)
甲苯摩尔百分数
液相
气相
81.5
3.3
5.1
81.4
6.9
8.1
81.1
12.18
12.18
81.3
18.2
15.4
81.4
22.1
17.2
81.8
28.4
19.5
82.7
37.5
23.0
84.4
53.3
28.4
85.6
60.5
31.5
91.1
78.5
41.7
93.4
84.0
47.3
95.6
87.6
52.6
101.2
92.9
66.5
103.6
95.6
73.8
106.7
97.7
83.6
107.5
98.2
85.4
Template:Clear
反应物
反应方程式
反应条件
作为配体
氧化亚铜 、六氟磷酸 Cu2 O + 2 HPF6 + 8 CH3 CN → 2 [Cu(CH3 CN)4 ]PF6 + H2 O
镍 、氟硼酸亚硝酰 Ni + 6 CH3 CN + 2 NOBF4 → [Ni(CH3 CN)6 ](BF4 )2 + 2 NO